Michael R. Harrison, M.D.
- Professor Emeritus of Surgery, Pediatrics, Obstetrics, Gynecology and Reproductive Sciences
- Director Emeritus, Fetal Treatment Center
Contact Information
Education
- 1961-65, Yale University, B.A.
-
1965-69, Harvard Medical School, M.D., Medicine
Residencies
- 1969-71, Massachusetts General Hospital, Resident, Surgery
- 1973-74, Massachusetts General Hospital, Senior Resident, Surgery
- 1974-75, Massachusetts General Hospital, Chief Resident, Surgery
Fellowships
- 1971-73, Laboratory of Immunology, National Institute of Allergy and Infectious Disease, Fellow, Allergy & Immunology (Peds)
- 1975-76, Rikshospitalet, Fellow, Pediatric Surgery
- 1976-78, Children's Hospital of Los Angeles, Fellow, Pediatric Surgery
Biography
Dr. Michael Harrison is Professor Emeritus of Surgery and Pediatrics and the Director Emeritus of the Fetal Treatment Center at UCSF. He graduated cum laude from Yale University and magna cum laude from Harvard Medical School. He completed his surgical training at the Massachusetts General Hospital in Boston and his pediatric surgery fellowship at Children's Hospital of Los Angeles. He is board certified in Surgery, Pediatric Surgery, and Critical Care.
Dr. Harrison and his surgery associates confine their surgical practice exclusively to children with special interest in fetal surgery, in repair of complex birth defects involving the chest, lung, abdomen, bowel, and bladder, and surgical care of children from birth through adolescence. Dr. Harrison and his associates, The Bay Area Pediatric Surgeons, do consultations and provide surgical care at Moffitt/Long Hospitals UCSF, California Pacific Medical Center, and Kaiser Permanente, San Francisco.
Dr. Harrison has a special interest in fetal surgery which he has pioneered with his colleagues at UCSF. For the past 18 years Dr. Harrison has studied the pathophysiology and natural history of a number of life-threatening fetal abnormalities including congenital diaphragmatic hernia, congenital cystic adenomatoid malformation of the lung, sacrococcygeal teratoma, fetal obstructive uropathy, and myelomeningocele. He has developed techniques for both in-utero open fetal surgery and endoscopic surgical repair (FETENDO Fetal Surgery)of many fetal abnormalities. In addition, Dr. Harrison's team has developed in-utero stem cell transplantation to treat immunodeficiencies, enzyme deficiencies, and hemoglobinopathies. Dr. Harrison leads the multidisciplinary UCSF Fetal Treatment Center Team that has developed an international reputation for treating complex birth defects before and after birth. (Referrals can be made to 1-800-RX-FETUS.)
Dr. Harrison, his wife Gretchen, and four children have lived in San Francisco for the past 20 years.
Research & Funding
- UCSF-Stanford Pediatric Device ConsortiumSponsor: FDASponsor ID: P50FD006424Funding Period:Sep 2018-Aug 2023Principal Investigator
- UCSF PEDIATRIC DEVICE CONSORTIUMSponsor: FDASponsor ID: P50FD003793Funding Period:Sep 2009-Aug 2019Principal Investigator
- Pain Control in the Nuss Procedure: A Prospective, Randomized Trial of Cryoanalgesia vs. Thoracic EpiduralSponsor: NIH/NICHDSponsor ID: R03HD090617Funding Period:Jul 2017-Jun 2019Principal Investigator
- Magnetic Duodeno-Ileal Bypass for Metabolic Syndrome in Rhesus MonkeysSponsor: NIH/NIDDKSponsor ID: R44DK112453Funding Period:Jun 2017-May 2018Principal Investigator
- Magnetic Mini Mover Device for the Treatment of Pectus ExcavatumSponsor: NIH/NHLBISponsor ID: R44HL134520Funding Period:Sep 2016-Aug 2017Principal Investigator
- Phase III Multicenter Study of Magnetic Mini-Mover for Pectus Excavatum G090006 1Sponsor: FDASponsor ID: R01FD003341Funding Period:Apr 2007-Mar 2015Principal Investigator
- MULTICENTER TRIAL OF FETAL MYELOMENINGOCELE REPAIRSponsor: NIH/NICHDSponsor ID: U10HD041669Funding Period:Jul 2001-Sep 2012Co-Principal Investigator
- PEDIATRIC CLINICAL RESEARCH CENTERSponsor: NIH/NCRRSponsor ID: M01RR001271Funding Period:Dec 1981-Mar 2007Co-Investigator
- FETAL WOUND HEALING: ROLE OF THE EXTRACELLULAR MATRIXSponsor: NIH/NICHDSponsor ID: R01HD025505Funding Period:Dec 1989-Feb 1998Co-Principal Investigator
- INDUCTION OF TOLERANCE IN MARROW TRANSPLANTATIONSponsor: NIH/NIAIDSponsor ID: P01AI029512Funding Period:Aug 1990-Jul 1995Co-Investigator
- CLINICAL TRIAL OF FETAL SURGERY FOR DIAPHRAGMATIC HERNIASponsor: NIH/NICHDSponsor ID: R01HD027308Funding Period:Sep 1992-Sep 1994Principal Investigator
- TRANSPLANTATION OF FETAL HEMATOPOIETIC STEM CELLSSponsor: NIH/NHLBISponsor ID: R01HL039875Funding Period:Feb 1988-Jun 1994Principal Investigator
Clinical Trials
- Study ID: NCT02431507Start Date: Sep 2015Estimated Completion Date: Dec 2021Condition(s): Sleep Apnea, Sleep Disorders
- Study ID: NCT02043392Start Date: May 2014Estimated Completion Date: Jun 2020Condition(s): Intestinal Anastomosis Complication
- Study ID: NCT00466206Start Date: Apr 2007Estimated Completion Date: Apr 2011Condition(s): Pectus Excavatum
Publications
MOST RECENT PUBLICATIONS FROM A TOTAL OF 500
- Lee WG, Evans LL, Harrison MR. Beyond the gut: spectrum of magnetic surgery devices. Front Surg. 2023; 10:1253728. View in PubMed
- Lee WG, Evans LL, Chen CS, Fuchs JR, Zamora IJ, Bruzoni M, Harrison MR, Muensterer OJ. Lessons Learned From the First-In-Human Compassionate Use of Connect-EA™ in Ten Patients With Esophageal Atresia. J Pediatr Surg. 2023 Sep 21. View in PubMed
- Chen C, Evans LL, Harrison MR. The Rearing of Maternal-Fetal Surgery: The Maturation of a Field from Conception to Adulthood. Clin Perinatol. 2022 Dec; 49(4):799-810. View in PubMed
- Holler AS, König TT, Chen C, Harrison MR, Muensterer OJ. Esophageal Magnetic Compression Anastomosis in Esophageal Atresia Repair: A PRISMA-Compliant Systematic Review and Comparison with a Novel Approach. Children (Basel). 2022 Jul 25; 9(8). View in PubMed
- Evans LL, Chen CS, Muensterer OJ, Sahlabadi M, Lovvorn HN, Novotny NM, Upperman JS, Martinez JA, Bruzoni M, Dunn JCY, Harrison MR, Fuchs JR, Zamora IJ. The novel application of an emerging device for salvage of primary repair in high-risk complex esophageal atresia. J Pediatr Surg. 2022 Dec; 57(12):810-818. View in PubMed
- View All Publications
In the News
- UCSF Surgical Innovations Program - Stacy Kim - August 31, 2018Michael Harrison, MD, Professor Emeritus of Surgery, Pediatrics, Obstetrics, Gynecology and Reproductive Sciences at the University of California, San Francisco (UCSF), Shuvo Roy, PhD, Professor of Bioengineering & Therapeutic Sciences at UCSF, and James Wall, MD, Assistant Professor of Surgery at Stanford University have been awarded a five-year $6.7 million Pediatric Device Consortia (PDC) P50 grant from the U.S. Food and Drug Administration (FDA). The UCSF-Stanford PDC is one of [...]


- UCSF Surgical Innovations - Heather R. Johnson - June 06, 2018Academic hospitals have all the makings for rich medical device development. They have physicians to identify surgical needs, researchers to test theories, bioengineers to design and create devices and patients to participate in clinical trials. However, the disconnect between engineering and surgical departments makes innovation a challenge. So does surgeons' all-consuming job Number One—treating patients. Providing the Infrastructure for Surgeons to Innovate Surgical Innovations, part of [...]

- UCSF Surgical Innovations - Stacy Kim - May 22, 2018On May 17, the Second Annual UCSF Engineering for Children's Health Symposium was held at Mission Bay Conference Center. Over one hundred clinicians, engineers, scientists, administrators, industry professionals, and government partners gathered to discuss groundbreaking research and developing technologies in pediatric medicine. The event commenced with opening remarks by Hanmin Lee, MD, Surgeon-in-Chief of the UCSF Benioff Children's Hospitals and Professor and Chief of the UCSF Division [...]

- UCSF Surgical Innovations - Stacy Kim and Richard Barg - September 28, 2017Results from the first-in-human clinical trial of a magnetic compression anastomosis device known as "Magnamosis" were recently reported on in the Journal of the American College of Surgeons. The study, led by Principal Investigator Michael Harrison, MD, is a prospective, single-center, first-in-human pilot clinical trial to evaluate the feasibility and safety of creating an intestinal anastomosis using the Magnamosis device. Magnetic compression anastomosis (magnamosis) uses a pair of [...]


- UCSF Surgical Innovations Program - Stacy Kim - August 29, 2017The Eunice Kennedy Shriver National Institute of Children Health & Human Development (NICHD) at the National Institutes of Health (NIH) has awarded pediatric surgeons Benjamin Padilla, MD, and Michael Harrison, MD, a two-year grant to study the use of cryoanalgesia as a novel method of pain control in the Nuss Procedure. The research team includes Sunghoon Kim, MD, and Jill Imamura-Ching, RN. Claire Graves, MD, played a key role in setting up the project and developing the grant application [...]


- Surgical Innovations Program - Stacy Kim - March 02, 2017A novel device to treat patients with obstructive sleep apnea was profiled in a recent story on ABC7/KGO News Bay Area. The technology was developed by Michael R. Harrison, M.D., Professor Emeritus of Surgery, Surgery, Pediatrics, Obstetrics, Gynecology and Reproductive Sciences and Director Emeritus of the UCSF Fetal Treatment Center. The surgery to implant the device was performed by Jolie Chang M.D., Assistant Professor in the divisions of General Otolaryngology, Salivary Gland Surgery and [...]


- UCSF Surgical Innovations Program - Elizabeth Gress - November 01, 2016The UCSF Department of Surgery's longstanding partnership with engineers to invent new medical technologies in response to patient needs was featured in a recent story by UC Berkeley journalism student Alex Orlando. The article profiles Richard Fechter, Principal Development Engineer at UCSF Medical Center and UCSF Benioff Children's Hospital, and long-time collaborator of Michael R. Harrison, M.D., Professor Emeritus of Surgery at UCSF, who pioneered the field of fetal surgery in the [...]

- Department of Surgery, Surgical Innovations - Richard Barg - July 08, 2016Pediatric surgeon Michael R. Harrison, M.D. and UC Berkeley engineer Phillip Messersmith, Ph.D. are collaborating on an NIH-Funded research project to improve glues used in fetal surgery procedures, notably those used to treat twin-to-twin transfusion syndrome. Dr. Harrision, widely acknolwedged as the "father of fetal surgery", is Director Emeritus of the Fetal Treatment Center and leads the research of the Harrison Lab. The full story was recently reported in UC Berkeley News. UC Berkeley [...]


- UCSF Surgical Innovations Program - October 23, 2015Two (2) Department of Surgery research teams were awarded seed funding totaling $40,000 to accelerate their medical device innovations. The Roboimplant device, an expandable rod for orthopaedic surgery applications, took top honors, with the Sentinel Bandage, a non-invasive technology for wound monitoring, taking second place. The awards were given at the UCSF Surgical Innovations Program's inaugural Shark Tank competition on September 24th at UCSF Mission Bay. Four teams of finalists pitched [...]

- UCSF Pediatric Surgery - September 17, 2015UCSF News reports on the discovery by researchers at UCSF Benioff Children's Hospital San Francisco that fetuses with enlarged ventricles, the fluid-filled cavities inside the brain, may be less likely than other fetuses to benefit from surgery in the womb to treat spina bifida. These findings were based on data from the 2011 Management of Myelomeningocele (MOMS) study, which demonstrated the potential benefit of treating spina bifida, a birth defect, in utero. The researchers found that [...]


- UCSF Surgical Innovations Program - March 17, 2015UC Berkeley News reports on the "smart bandage," a device in development by a team of surgeons and scientists at UCSF and UC Berkeley that uses electrical currents to detect early tissue damage from pressure ulcers, or bedsores, well before they are visible to the human eye. The technology offers the possibility of early intervention to prevent progression of the wound. David M. Young, MD, Professor in the Division of Plastic and Reconstructive Surgery, is leading the clinical development of [...]

- UCSF Pediatric Surgery - May 06, 2013"Innovation in medicine is driven by need, but also by the market," said Dr. Michael R. Harrison, the director emeritus of the Fetal Treatment Center and the director of the Pediatric Device Consortium, both at the University of California, San Francisco. "Big markets have lots of folks developing devices, but small markets like the pediatrics market don't."
- UCSF Pediatric Surgery - November 02, 2012Michael R. Harrison, MD, founder and director emeritus of the Fetal Treatment Center at the UCSF Benioff Children's Hospital, was recognized last week for his contributions to life-saving fetal surgery with the Ronald McDonald House Charities Medical Award of Excellence.

- UCSF Pediatric Surgery - August 19, 2012Surgeons at the UCSF Benioff Children's Hospital in San Francisco are using magnets to reshape the breastbones of children who suffer from Sunken Chest Syndrome. The technique is undergoing phase 3 clinical trials, but the doctors hope to prove that long term magnetic force is as effective and less painful than conventional surgery.
- UCSF Pediatric Surgery - July 30, 2012A new method for repairing Pectus Exacavatum using magnets and an external brace, developed by Michael Harrison, a pediatric surgeon at the University of California, San Francisco's Benioff Children's Hospital, could provide an alternative to the surgery.
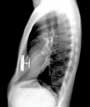
- UCSF Pediatric Surgery - January 27, 2012UCSF has received about a million dollars since 2009. That money has supported the development of tools to treat scoliosis, kidney failure and sunken chest, among other conditions. The pectus, or sunken chest device, is in clinical trials.

- UCSF Pediatric Surgery - November 01, 2011The UCSF "D'Vice Squad," a group of innovators from across the Bay Area, has drawn from diverse disciplines over the last two years to develop medical devices for children. Now the squad's hard work has been rewarded with a $1 million grant from the U.S. Food and Drug Administration (FDA) to expand its work over the next two years.

- UCSF Fetal Treatment Center - February 09, 2011For years, surgeons have been seeking ways of operating on babies in the womb, reasoning that medical abnormalities are easier to address while the fetus is still developing. Now, for the first time, a large clinical trial has shown that fetal surgery can also benefit infants with non life-threatening conditions. The eight-year study reported in the New England Journal of Medicine, found that babies born with myelomeningocele, the most common form of spina bifida, a debilitating spinal [...]

- UCSF Pediatric Surgery - November 02, 2010UCSF announces the formation of the Institute for Fetal and Neonatal Health symposium brings together clinicians and basic scientists involved in different aspects of development and fetal intervention.

- UCSF Pediatric Surgery - August 17, 2010Stopping curvature of the spine in kids usually requires a series of painful operations to implant rods and screws to adjust the spine. Even then, the results are often less than perfect. However, a medical team at UCSF has developed a technique using magnets that promises to do away with so many surgeries.
- UCSF Pediatric Surgery - April 07, 2010Magnets have a brand new use. They are being tested in a new procedure to correct a rare birth defect , Pectus Excavatum, in a Los Gatos boy. Dr. Kim Mulvihill reports.
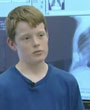
- UCSF Department of Surgery - November 19, 2009In our efforts to establish a distinguished professorship in honor of Michael R. Harrison, M.D, an extremely generous donor has called for a challenge. The anonymous donor has pledged a gift of $500,000 in the form of a match. Our goal must be met by Dec 31, 2009.

- UCSF Pediatric Surgery - October 12, 2009Michael Harrison M.D., a renowned pioneer in fetal and pediatric surgery, Professor Emeritus of Surgery and Pediatrics, and Director Emeritus of the Fetal Treatment Center at UCSF, has been elected to the prestigious Institute of Medicine (IOM), one of the U.S. National Academies. Membership in the IOM reflects "the height of professional achievement and commitment to service" and is reserved for those at the pinnacle of their field.

- UCSF Pediatric Surgery - January 15, 2008Dr. Harrison helped change the face of medicine. Today, UCSF is building a medical complex where scientific innovations become life-saving treatments ...

- UCSF Pediatric Surgery - November 08, 2007UCSF has created a groundbreaking new procedure to treat chest deformities in children and teens. This minimally invasive procedure is already transforming one 14-year-old's life.

